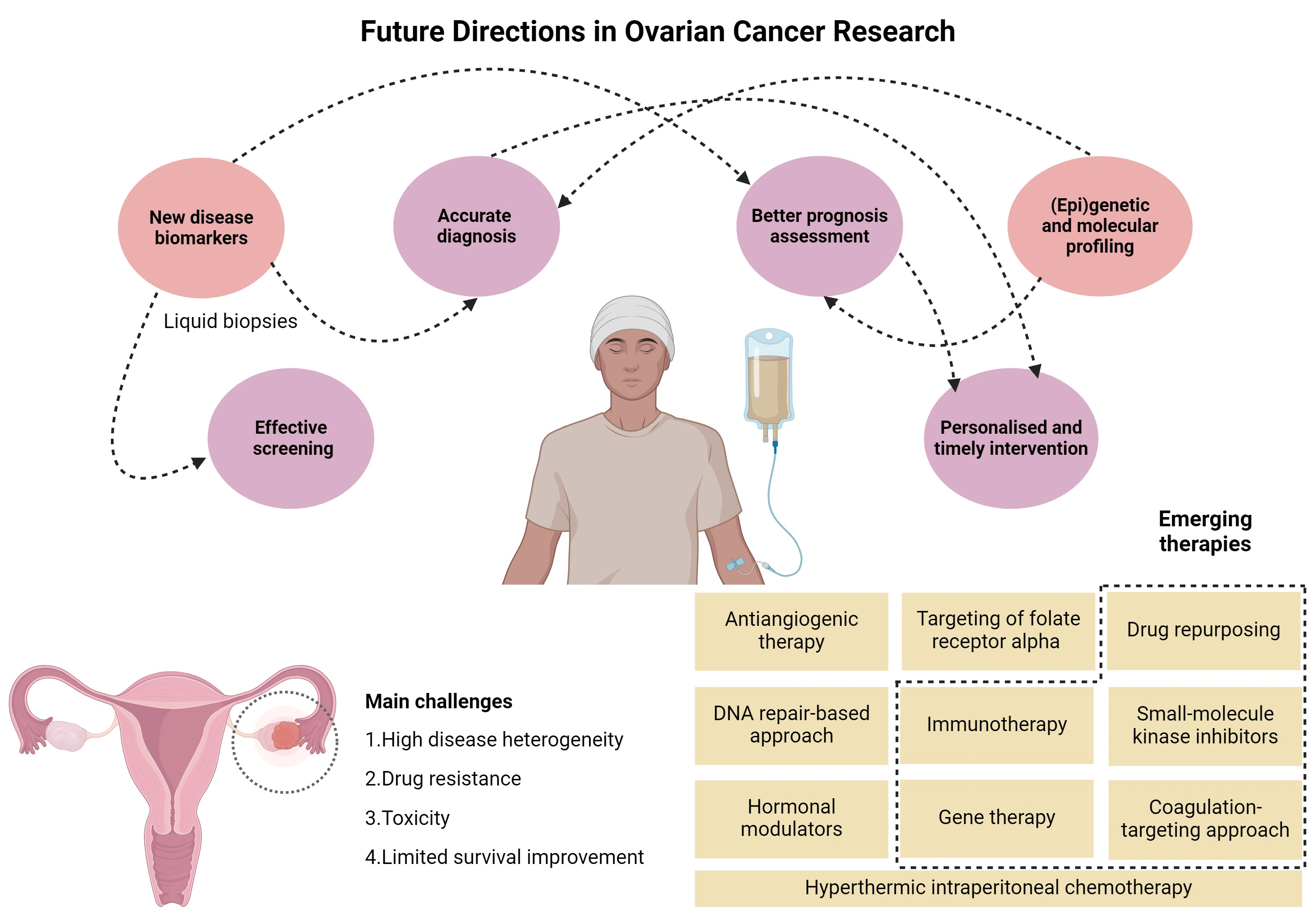
Ovarian cancer is a serious condition that occurs when abnormal cells in the ovaries or fallopian tubes grow uncontrollably, spreading to surrounding tissues. This aggressive form of cancer often goes undiagnosed in its early stages, making it particularly challenging to treat effectively. Ovarian Cancer Drug Pipeline Analysis According to the World Cancer Research Fund International, ovarian cancer ranks as the eighth most common cancer in women globally. The disease is particularly prevalent among older women, with its incidence increasing with age.
In recent years, the development of new therapies and drug treatments for ovarian cancer has accelerated, driven by advances in medical research and clinical trials. Ovarian Cancer Drug Pipeline Analysis As a result, the ovarian cancer drug pipeline is becoming increasingly diverse, with numerous candidates showing promise for improving survival rates and quality of life for patients. Several drugs under clinical development are targeting key aspects of ovarian cancer biology, from immune checkpoints to gene therapies.
Get a Free Sample Report with a Table of Contents: https://www.expertmarketresearch.com/clinical-trials/ovarian-cancer-drug-pipeline-analysis/requestsample
ovarian cancer drug pipeline, exploring the current landscape of clinical trials, emerging therapies, market trends, and the key players involved in the development of new treatments.
Ovarian Cancer Drug Pipeline Analysis Overview
The ovarian cancer drug pipeline consists of various stages of clinical development, ranging from preclinical studies to late-phase trials. These drugs are designed to address the complex biology of ovarian cancer, targeting factors such as tumor growth, immune evasion, and metastasis.
1. Drug Discovery and Preclinical Studies
Early-stage drug discovery for ovarian cancer focuses on identifying compounds that can disrupt key signaling pathways involved in the disease. Researchers explore small molecule inhibitors, monoclonal antibodies, and other biologics that can target the genetic mutations and environmental factors driving tumor growth.
Several companies are also investigating gene therapy and cell-based treatments to introduce new genetic material into cancer cells, either to repair damage or to disrupt abnormal pathways.
Read Full Report with Table of Contents: https://www.expertmarketresearch.com/clinical-trials/ovarian-cancer-drug-pipeline-analysis
2. Clinical Development Phases
Once promising compounds are identified, they progress through clinical trials, which are typically divided into three phases:
- Phase I trials primarily assess safety and dosage.
- Phase II trials evaluate the effectiveness and further investigate safety.
- Phase III trials compare the new drug to existing treatments, demonstrating its ability to improve patient outcomes.
After Phase III, if a drug shows efficacy and safety, it may be approved for general use by regulatory bodies like the FDA and EMA.
3. Regulatory Approvals and Market Launch
Once regulatory bodies approve new treatments for ovarian cancer, they become available to patients through hospitals and clinics. Ongoing monitoring and additional studies ensure that these drugs remain effective and safe for long-term use.
Ovarian Cancer Drug Pipeline Dynamics
The ovarian cancer drug pipeline is shaped by several dynamic factors, including technological advances, regulatory processes, and market demands. Let’s explore the key dynamics affecting the pipeline:
1. Targeted Therapies and Precision Medicine
Targeted therapies aim to block specific molecules involved in tumor growth. These drugs are designed to interfere with the molecular pathways that promote cancer cell survival and proliferation. Precision medicine, which tailors treatments based on an individual’s genetic profile, has been a game-changer in oncology, including ovarian cancer. Targeted treatments are increasingly becoming a cornerstone of ovarian cancer therapy, allowing for more personalized and effective options.
2. Immunotherapy
Immunotherapy is another promising area of research for ovarian cancer. This approach aims to harness the patient’s immune system to fight the cancer. Immune checkpoint inhibitors, such as PD-1 and PD-L1 inhibitors, are gaining traction in clinical trials. These therapies work by blocking the immune system’s brakes, allowing T-cells to recognize and destroy cancer cells more effectively.
3. Combination Therapies
As single-agent therapies often fall short in treating advanced ovarian cancer, combination therapies are being explored. By combining different classes of drugs—such as chemotherapy with immunotherapy or targeted therapy—researchers aim to enhance treatment efficacy and overcome drug resistance, a common challenge in ovarian cancer treatment.
4. Regulatory Approvals
Several ovarian cancer treatments are progressing rapidly through clinical trials, and the regulatory approval process is a critical component of the pipeline. Regulatory agencies like the FDA and EMA are offering accelerated approval pathways for promising therapies, particularly for rare and aggressive cancers like ovarian cancer.
5. Epidemiological Factors
The prevalence and mortality rates of ovarian cancer vary significantly across different regions, which impacts drug development strategies. The ongoing rise in the global geriatric population is expected to drive the demand for more effective treatments for age-related conditions such as ovarian cancer.
External Ovarian Cancer Drug Pipeline Trends
Several external factors are influencing the ovarian cancer drug pipeline:
1. Technological Advancements
Recent technological innovations, such as next-generation sequencing and CRISPR gene-editing technologies, are accelerating the discovery of new targets for ovarian cancer therapy. These breakthroughs are making it possible to understand the genetic basis of ovarian cancer better, opening the door to the development of more precise and targeted therapies.
2. Increased Funding for Cancer Research
Cancer research, including ovarian cancer, continues to receive substantial funding from both public and private sectors. Governments, foundations, and pharmaceutical companies are investing heavily in drug development and clinical trials, which is helping to fuel innovation in the ovarian cancer drug pipeline.
3. Collaborations and Partnerships
Pharmaceutical companies and research institutions are forming strategic collaborations to share knowledge, resources, and expertise. These partnerships enable faster drug development and a broader range of treatment options for ovarian cancer.
Ovarian Cancer Drug Pipeline Segmentation
The ovarian cancer drug pipeline can be segmented by drug class, stage of development, and mechanism of action. Here’s a closer look:
1. By Drug Class
- Chemotherapy: Chemotherapeutic agents like carboplatin and paclitaxel remain foundational in treating ovarian cancer, although they are often associated with significant side effects.
- Targeted Therapy: Drugs like PARP inhibitors (e.g., Olaparib) are designed to target specific cancer cell vulnerabilities, offering a more tailored approach to treatment.
- Immunotherapy: The introduction of immune checkpoint inhibitors is offering new hope, with several under investigation for ovarian cancer.
2. By Stage of Development
- Early-Stage Development: These drugs are undergoing preclinical testing or early-phase clinical trials, with early results showing promise.
- Late-Stage Development: These treatments are undergoing Phase II and Phase III trials, where their efficacy and safety are being rigorously tested.
3. By Mechanism of Action
- Angiogenesis Inhibitors: These drugs prevent tumors from developing blood vessels, starving them of the oxygen and nutrients they need to grow.
- DNA Repair Inhibitors: Targeting mechanisms involved in DNA repair, such as PARP inhibitors, aims to make cancer cells more susceptible to damage.
Ovarian Cancer Drug Pipeline Growth
The ovarian cancer drug pipeline is experiencing significant growth, driven by the need for better treatment options and advancements in drug discovery. The growth of the pipeline can be attributed to:
- Increased Research and Clinical Trials: Research institutions and biotech companies are rapidly advancing the pipeline, with more drugs in clinical trials than ever before.
- Collaborations and Partnerships: Public-private partnerships and collaborations between pharma companies are speeding up the development of new ovarian cancer therapies.
- Emerging Market Demand: Growing demand for innovative treatments, particularly in developing markets, is further driving the pipeline’s expansion.
Recent Ovarian Cancer Drug Pipeline Market
The ovarian cancer drug pipeline has seen several promising developments in recent years. Notable trends in the market include:
- The Emergence of PARP Inhibitors: Drugs like Olaparib and Rucaparib have shown strong efficacy in patients with BRCA mutations.
- Immunotherapy Advancements: Immune checkpoint inhibitors such as nivolumab and pembrolizumab are under investigation for use in ovarian cancer, showing potential in early trials.
Ovarian Cancer Drug Pipeline Analysis
The ovarian cancer drug pipeline is at a critical juncture, with promising therapies advancing through various stages of development. However, challenges such as drug resistance, side effects, and high treatment costs remain. The future of the pipeline will depend on continued innovation and regulatory approvals, alongside the exploration of novel therapeutic combinations.
COVID-19 Impact Analysis
The COVID-19 pandemic had a significant impact on clinical trials, including those for ovarian cancer drugs. The disruption of clinical trial timelines, delays in patient recruitment, and limitations on in-person visits affected many studies. However, the pandemic has also highlighted the need for more efficient drug development processes, driving innovation in digital health solutions and remote patient monitoring.
Key Players in Ovarian Cancer Drug Pipeline
- AstraZeneca: Known for its work on immune-oncology drugs and targeted therapies for ovarian cancer.
- Daiichi Sankyo Co., Ltd.: Developing innovative therapies in the field of targeted treatments and immunotherapies for ovarian cancer.
- GlaxoSmithKline: Focused on advancing treatments in immuno-oncology and other therapeutic areas for ovarian cancer.
FAQ
1. What are the main treatments for ovarian cancer?
Current treatments include chemotherapy, targeted therapies, and immunotherapy. Advances in these treatments are focused on improving efficacy and reducing side effects.
2. What are the most promising drug candidates for ovarian cancer?
PARP inhibitors, immune checkpoint inhibitors, and angiogenesis inhibitors are among the most promising therapies in development.
3. How is the ovarian cancer drug pipeline progressing?
The pipeline is expanding with multiple drugs entering clinical trials and showing promise for improving treatment outcomes.
4. What is the role of precision medicine in ovarian cancer treatment?
Precision medicine aims to tailor treatment based on genetic and molecular profiling, offering more effective, individualized options for patients.